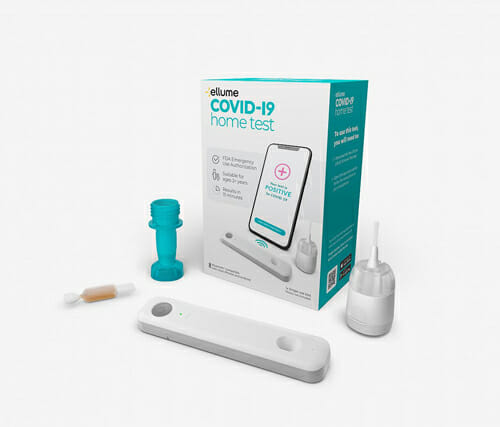
The Food and Drug Administration’s approval of a new over-the-counter, at-home COVID-19 diagnostic test has won praise from the home care community and lawmakers.
“Homebound patients have had very limited access to COVID-19 testing,” William A. Dombi, president of the National Association for Home Care & Hospice, told the McKnight’s Home Care Daily on Tuesday. “The availability of a low-cost home test will be very helpful in the proper diagnosis and treatment for those individuals.”
A LeadingAge spokeswoman agreed. “Broad-scale, reliable at-home testing will be a boon for care in the home and community, as well as for all aging services,” she told McKnight’s.
Sen. Lamar Alexander (R-TN), chairman of the Senate Health Committee, also welcomed approval of the test, which he called a “genuine breakthrough in COVID-19 testing.” Alexander made his remarks on Tuesday following FDA approval of the test.
The test is a result of the National Institute of Health’s “Shark-Tank”-like initiative announced last April to accelerate COVID-19 testing. Test cartridges contain a single-use, digital fluorescent immunoassay antigen test that returns results in 15 minutes or less. Consumers can purchase the low-cost test over-the-counter without a prescription. Ellume, a developer and manufacturer of digitally enabled diagnostic products, created the test.
“This is the result of the extraordinary efforts of the National Institutes of Health’s initiative, which has produced 22 new ways to create tens of millions of diagnostic tests in the last 8 months,” Alexander said.




