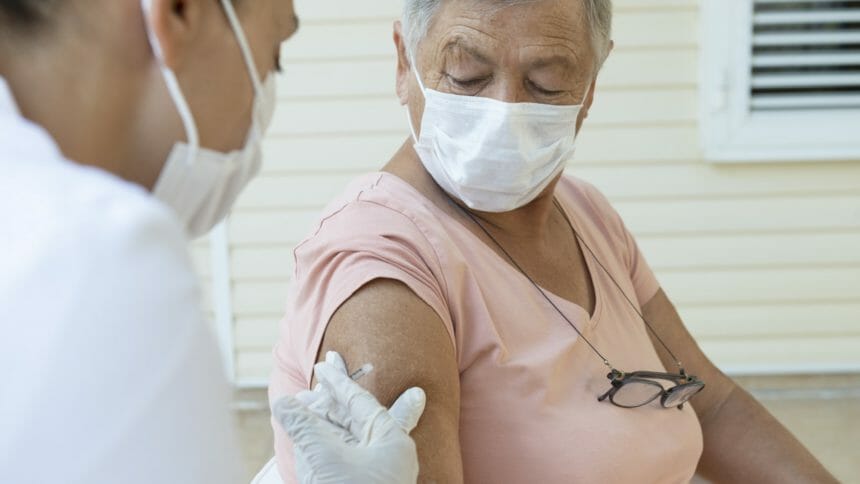
Even before another federal agency’s panel approved booster shots for seniors, the wheels were in motion at some home care companies to get COVID-19 boosters into the arms of their clients. Among them is St. Paul’s Program for All-Inclusive Care for the Elderly in San Diego, which is aiming to start vaccinating its 1,600 participants as early as Monday.
St. Paul’s PACE CEO Cheryl Wilson told McKight’s Home Care Daily she ordered 1,000 doses of the Pfizer vaccine earlier this week in anticipation of The Food and Drug Administration and the Centers for Disease Control & Prevention approving the boosters by the end of the week.

“The only thing that would hold us up from doing this on Monday would be not getting the doses from Pfizer,” Wilson said. “If we get the order delivered on Friday, we are ready. We have nurses on standby. We know how to do this.”
Wilson said St. Paul’s PACE has already given first and second vaccinations to 99% of its clients through a program that administered shots to them in their homes or at a PACE center. The program will follow a similar strategy with the boosters.
Authorizations
Late Thursday afternoon, a CDC scientific advisory panel approved the boosters for people over age 65. The CDC must still green light the boosters. On Wednesday, the FDA authorized Pfizer booster vaccinations to Americans 65 and older, younger adults with underlying health conditions and workers in jobs that put them at risk of contracting COVID-19.
Plans to give booster vaccinations have been in the works for more than a month, but last Friday an FDA advisory panel scaled back a plan by the Biden administration that would have allowed most adults to get a booster dose of the vaccine.
Not so fast
While Wilson and her team are ready to go full speed ahead with the boosters, some agencies that previously had vaccination programs in place aren’t ready to provide boosters.
Lewiston, ME-based Androscoggin Home Healthcare & Hospice delivered hundreds of COVID-19 vaccinations to seniors in their homes earlier this year. But the agency’s chief clinical officer, Leann Sebrey, told McKnight’s Home Care Daily in an email her agency doesn’t have enough freezer space to store the Pfizer vaccine at this time and is waiting for clarity regarding approval of the Moderna and Johnson & Johnson boosters.

“We need more information and guidance to do this safely,” Sebrey wrote.
San Francisco-based On Lok PACE is also waiting for FDA and CDC approval of Moderna’s boosters. Once that happens, the PACE program should be able to get the additional vaccine into the arms of its nearly 1,700 participants fairly quickly. Thomas Yi, On Lok’s Director of Pharmacy Services, told McKnight’s Home Care Daily the program’s experience administering the first vaccine should make this process easier.
“It would just be the case where we need to make sure that we have the vaccine in stock and have our teams ready to go, whether it’s bringing them into the clinic or going to their homes to vaccinate them,” Yi said.
Meanwhile, Long Beach, CA-based healthcare plan SCAN Health told McKnight’s Home Care Daily it won’t be providing any in-home vaccination to its Medicare Advantage clients as it did in the spring with the first shots. This time around it will be connecting clients to California’s public and clinic management system, My Turn, to coordinate in-home boosters for seniors through their counties.




